Effective October 3, 2022, Abbot multi-collect kits [ITM-1093897] will be discontinued and replaced with Alinity m Collection kit [ITM-1190747].
STI testing
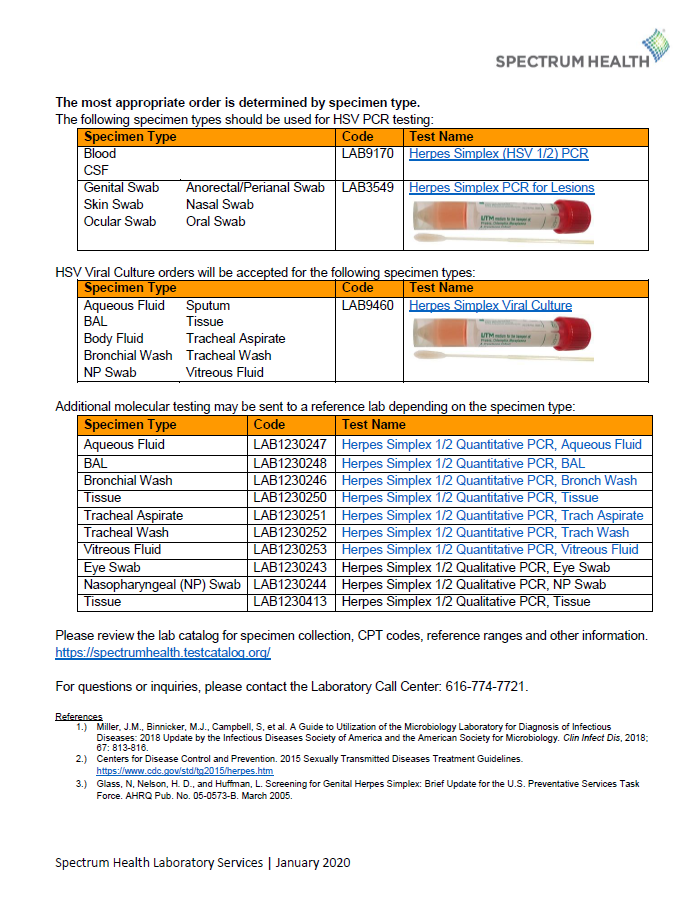
As of April 5th, 2022, Herpes Simplex viral PCR testing will transition from being performed in the Spectrum Health Molecular department to the Microbiology department. There are no changes to the acceptable specimen sources for this test (cerebrospinal fluid and blood plasma specimens). Benefits of the new Microbiology testing method include:
• The new test method (DiaSorin Simplexa® HSV PCR) is FDA-cleared for testing spinal fluid specimens with a validated modification for testing plasma specimens. The prior method was a lab-developed test for both specimen types.
• Especially for pediatric patients, collecting sufficient specimen volumes can sometimes be challenging. The new method requires a minimum of 0.05 mL specimen per run whereas the prior method required 0.2 mL specimen.
• The frequency of testing will increase from typically one run per day to testing being performed at several times each day.
Self-collected vaginal swabs may be used for testing as opposed to provider-collected swabs. Self-collected swabs are supported by current clinical guidelines as recent studies have shown their equivalence, if not superiority, in quality and their association with increased patient satisfaction.
NOTE: Self-collection must still take place in a healthcare setting and is not approved for at-home collection.
Effective Wednesday, November 13, 2019, the new Mycoplasma genitalium (mgen) assay is now available using the Aptima target nucleic acid amplification test (NAAT) for the qualitative detection of ribosomal RNA (rRNA) from Mycoplasma genitalium to aid in the diagnosis of M. genitalium urogenital infections in male and female patients.