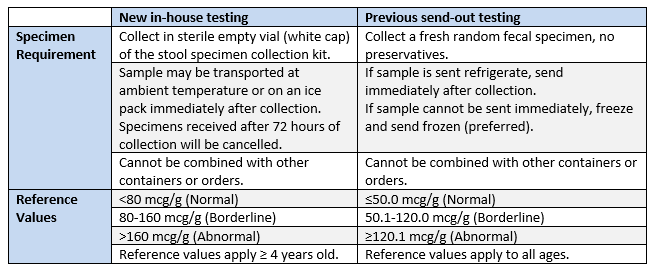
Effective May 18, 2020, Spectrum Health Regional Laboratory is pleased to be offering in-house COVID-19 serology testing, with initial availability 1,000 tests per day.
What is Serology Testing?
• Serology testing measures the body’s immune response to COVID-19 infection in the form of antibody production against the SARS-CoV-2 virus.
• There is a single COVID-19 serology order available in Epic, however, the Spectrum Health laboratory performs two versions of antibody testing to increase specificity and avoid reporting false positive results. An initial screen will be used to measure total antibody (IgA, IgM, and IgG), and positives will be confirmed by a second method that is specific to IgG.
• Specificity is critical when the expected prevalence in a community is low. This serology testing is not expected to cross-react with other circulating coronaviruses that cause the common cold.
• A positive IgG result indicates previous infection with COVID-19, but does not indicate immunity or protection against future infection.
• This test should not be used to detect acute COVID-19 disease. Symptomatic patients suspected to have acute COVID-19 infection should be tested using a molecular assay.
• Whether positive or negative for the presence of COVID-19 antibodies, serology testing results do not support easing of behaviors such as social distancing, wearing masks, or hand hygiene.