As of January 10th, 2024, the vitamin B1 (thiamine) assay has been updated to align with laboratory best practices. Patients being monitored may have higher vitamin B1 levels as compared to samples tested on the previous assay.
Toxicology
Effective Wednesday, November 29, 2023, the in-house test, Pyruvic Acid, will be made inactive. Recommended alternative testing is Pyruvic Acid, Blood [LAB1231461] which is referred to Mayo Clinic Laboratories. Please review the lab catalog for information on new specimen requirements and updated reference range information. Due to the update in reference ranges, this may disrupt trending data.
The test Lactate/Pyruvate Ratio will also be inactivated with no alternative testing. If you require assistance with obtaining the ratio, please Contact Us.
Effective February 28th, 2023, Vitamin D 25-Hydroxy Level (25-OH Vitamin D) [LAB535] will be replaced by 25-Hydroxyvitamin Level D2 and D3 [LAB1230925]. Send out testing for 25-Hydroxyvitamin D2 and D3, Serum [LAB1230428] to Mayo will be discontinued. The testing methodology will change from an immunoassay to liquid chromatography-mass spectrometry.
The new test will include concentrations for
- 25-hydroxyvitamin D2
- 25-hydroxyvitamin D3
- 25-hydroxyvitamin D Total
Effective November 21, 2022, the following changes will be made to Meconium Drug Testing (LAB479).
- The cutoff for positivity for the opiate drug class (codeine, morphine, hydrocodone, hydromorphone, oxycodone, and oxymorphone) will increase to 20 ng/g from 10 ng/g.
- The format of reporting will be updated to include discrete reporting fields for all tested analytes. Positive results will no longer be denoted via comments.
As of July 19, 2022, in alignment with recommendations from the Center for Disease Control and Prevention (CDC) the reference range for blood lead testing has been updated from 5 mcg/dL to 3.5 mcg/dL.
This applies to both: Lead, Blood Level [LAB98] and Lead Screen Filter Paper [LAB2111119].
In addition, at this time the low end of reporting for Lead Screen Filter Paper [LAB2111119] has increased from 1 mcg/dL to 2 mcg/dL.
Specimen Collection Updates – July 2021
The following information was updated in the Spectrum Health Laboratory Catalog.
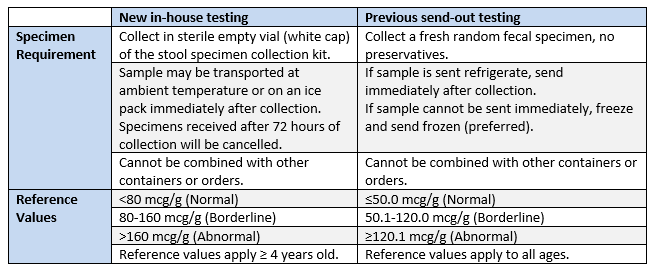
As of February 12, 2020 testing for fecal calprotectin will be brought in-house.
As result Calprotectin, Feces (LAB3290) will be replaced with Calprotectin, Feces (LAB1230580).
Please note the following differences between the new and previous test in terms of specimen requirements and reference ranges.
Questions may be directed to the Toxicology Laboratory using the “Contact Us” link above.
Effective Wednesday, December 18, 2019, Cystatin C will change from a sendout reference test performed by Mayo Clinic Laboratories to an in-house test performed by Spectrum Health Regional Laboratory. This test will be performed in the Toxicology Laboratory and will include a new reference range (please see link in Test Information below).
Questions may be directed to Toxicology via the “contact us” link above.
TEST INFORMATION
Cystatin C – Epic Code #LAB3226, Interface #11631, CPT #82610