The following information was updated in the Lab Catalog.
All changes are for Corewell Health Laboratories West.
The following information was updated in the Lab Catalog.
All changes are for Corewell Health Laboratories West.
Effective Thursday, April 18, 2024, Corewell Health Laboratory will discontinue the send out test Hepatitis A IgG Antibody, Serum (Mayo Test ID: HAIGG). This test has been made obsolete by Mayo Clinic Laboratories. We recommend to our healthcare providers the following in-house testing:
1.Hepatitis A Total Antibody (LAB1230596): intended for the clinical laboratory diagnosis of acute or past hepatitis A virus infection in persons with signs or symptoms of hepatitis and in persons at increased risk for hepatitis A infection, to identify HAV susceptible individuals and to determine the presence of an antibody response to HAV in vaccine recipients.
2.Hepatitis A Antibody, IgM (LAB7980): intended for use as an aid in the laboratory diagnosis of an acute or recently acquired hepatitis A virus infection.
The following information was updated in the Lab Catalog.
Effective Tuesday, February 20, 2024, the policy for home collected specimen drop offs for Outpatient Laboratories (“draw sites”) has been updated.
For all Corewell Health West Michigan locations: after registration, patients or their designated person will verify the specimen at the laboratory. Laboratory team members will verify the specimen is properly labeled and ensure they have everything needed to be able to test the specimen before the patient or their designated person leaves the building. This does not include specimens collected by a home health nurse or provider.
This will standardize our patient flow when arriving with a home collected specimen and will reduce the need for recollection due to missing information or improper collection.
When specimen collection orders are placed, please notify patients of the change to this process.
RELATED RESOURCES
Effective February 7, 2024, at 7:30 AM, Corewell Health West Laboratories will go live with a new reference range for the calculated Anion Gap (AG). The AG reference range will be adjusted to align with recent reference range studies.
The following will be the new normal reference ranges for AG:
Anion Gap: 5 – 14 mmol/L
The following information was updated in the Lab Catalog.
All changes are for Corewell Health Laboratories West.
As of January 26, 2024, for the following tests, the testing platform has changed from EliA Fluorescence Enzyme Immunoassay (FEIA) from Thermofisher to Multiplex Flow Immunoassay from Biorad. Please review the lab catalog for any changes to collection information or reference ranges.
• Anti-dsDNA Antibody
• Cyclic Citrullinated Peptide (CCP) Antibody
• Celiac Antibody Cascade
• Tissue Transglutaminase Antibody
• Gliadin Antibodies
Written by Yasel Fleitas Alvarez, Ph.D., Chemistry Clinical Advisor, Corewell Health Reference Laboratory West, Michigan Pathology Specialists.
This January we are celebrating the National Thyroid Awareness Month. In United States of America, it is estimated that approximately 20 million people have thyroid disease and most importantly, according to the American Thyroid Association (ATA) as many as 60% of people suffering from a thyroid disorder are not aware they have it.The thyroid is a butterfly shaped-gland located at the front of the neck that produces and release thyroid hormones (See Figure 1).
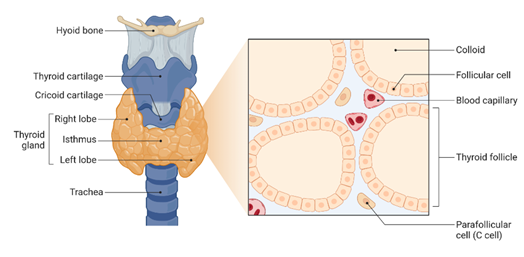
Figure 1. Thyroid Gland Anatomy and Histology
It regulates important physiological functions as:
Thyroid disease can present in two main forms:
Confirmation or exclusion of thyroid disease requires a clinical examination combined with biochemical determination of thyroid hormones (TH) and thyrotropin (TSH)concentrations.
In this blog we discuss the best practices for ordering thyroid function tests for the initial screening of thyroid disease at Corewell Health.