Spectrum Health Immunochemistry Laboratory is pleased to announce 5 new allergen tests and the addition of Tryptase. The following allergens are now available:
Internal Medicine
Effective December 9, 2020, the following insect allergen components will be available:
◊ Allergen Honey Bee Component Panel – EPIC #LAB1230747, Interface #1230747, CPT 86008
◊ Allergen Common Wasp (Yellow Jacket) Component Panel – EPIC #LAB1230752, Interface #1230752, CPT 86008
◊ Allergen Paper Wasp Component rPol d 5 – EPIC #LAB1230754, Interface #1230754, CPT 86008
◊ Cross-reactive Carbohydrate Determinant (CCD) – EPIC #LAB1230755, Interface #1230755, CPT 86008
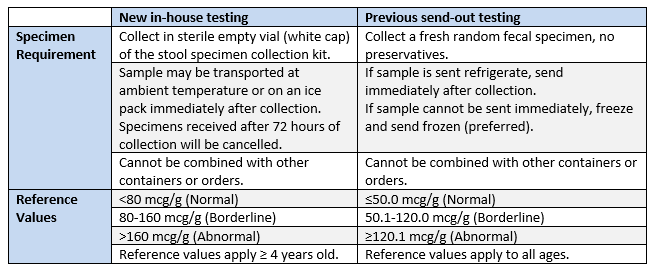
As of February 12, 2020 testing for fecal calprotectin will be brought in-house.
As result Calprotectin, Feces (LAB3290) will be replaced with Calprotectin, Feces (LAB1230580).
Please note the following differences between the new and previous test in terms of specimen requirements and reference ranges.
Questions may be directed to the Toxicology Laboratory using the “Contact Us” link above.
Effective December 11th, 2019, Spectrum Health Regional Laboratory’s Immunochemistry laboratory will be upgrading the following tests from the Immunoassay Vidas platform to the Multiplex flow immunoassay Bioplex 2200 platform.
All tests will continue to have the same collection instructions, processing instructions, rejection criteria, specimen stability, turn-around-time, and qualitative reference ranges of negative.
Herpes Simplex Virus (HSV) IgM Antibody Testing Update
As of November 25th 2019 the following tests for Herpes Simplex Virus (HSV) IgM antibody testing will be obsoleted.
Herpes IgM Antibody Screen – LAB3576
Herpes Simplex Virus (HSV) Antibody Screen, IgM, by EIA, Serum – LAB3578
Herpes Simplex Panel – LAB1230539
Protein Electrophoresis Testing Update
In December, serum and urine protein electrophoresis testing will be simplified and standardized. The only testing available will be:
Protein electrophoresis, serum, IFE if indicated
Protein electrophoresis, random urine, do IFE if indicated
Protein electrophoresis, 24 hour urine, do IFE if indicated
In addition, on December 5, 2019, a new panel will be added which reflects expert recommendations for first line testing for monoclonal gammopathy, including plasma cell myeloma and most cases of amyloidosis, called “Monoclonal Gammopathy Screen”